*By Dr. Devan
Introduction
The human pancreas is a dual-function organ, serving both exocrine and endocrine roles. Among its endocrine components, the islets of Langerhans are critical for glucose regulation, producing hormones such as insulin, glucagon, somatostatin, and pancreatic polypeptide. The destruction or dysfunction of insulin-producing β-cells is the hallmark of diabetes mellitus, particularly type 1 diabetes, where autoimmunity eliminates these cells, and type 2 diabetes, where β-cell exhaustion contributes to disease progression.
For decades, researchers believed that adult human β-cells had very limited regenerative capacity. However, recent discoveries have shifted this paradigm, highlighting molecular pathways that could restore or replace β-cell mass. Among these, Neurogenin-3 (Ngn3) has emerged as the master regulator of islet cell development and regeneration. Ngn3 is a transcription factor essential for pancreatic endocrine lineage determination during embryogenesis, and fascinatingly, under certain stress and dietary interventions, it appears to be reactivated even in adult life, offering hope for diabetes reversal.
This essay explores the biology of Ngn3, its role in embryonic pancreatic development, experimental evidence of its reactivation in adulthood, and the groundbreaking implications for regenerating insulin-secreting islet cells of the pancreas.
The Biology of Neurogenin-3
1. Definition and Structure
Neurogenin-3 is a basic helix-loop-helix (bHLH) transcription factor encoded by the NEUROG3 gene. It functions as a master switch that drives multipotent pancreatic progenitor cells toward an endocrine fate.
2. Role in Embryogenesis
During embryonic development, pancreatic progenitor cells can differentiate into either exocrine (acinar and ductal) or endocrine (islet) lineages.
The expression of Ngn3 marks a point of no return toward the endocrine pathway.
Ngn3-positive progenitors give rise to all islet cell types:
β-cells (insulin-secreting), α-cells (glucagon-secreting), δ-cells (somatostatin-secreting)
PP-cells (pancreatic polypeptide), ε-cells (ghrelin-secreting)
Without Ngn3, the endocrine pancreas fails to form. Knockout studies in mice confirm that Neurog3-null animals lack pancreatic islets entirely, highlighting its indispensable role.
Ngn3 in the Adult Pancreas: Dormant but Reactivatable. In healthy adults, Ngn3 expression is typically silenced after birth. Mature β-cells maintain their identity without needing the embryonic transcriptional program. However, under certain conditions of stress, injury, or metabolic intervention, dormant progenitor cells in pancreatic ducts can be induced to re-express Ngn3.
Sources of Reactivated Ngn3
Pancreatic ductal epithelium – cells lining the ducts can revert to a progenitor-like state.
Acinar-to-ductal metaplasia – acinar cells may undergo partial reprogramming.
Endocrine progenitors retained in niches – remnants of developmental programs may be reawakened.
This reactivation opens the possibility of generating new insulin-producing cells even in adulthood.
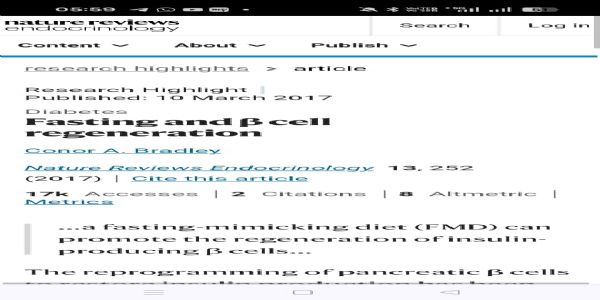
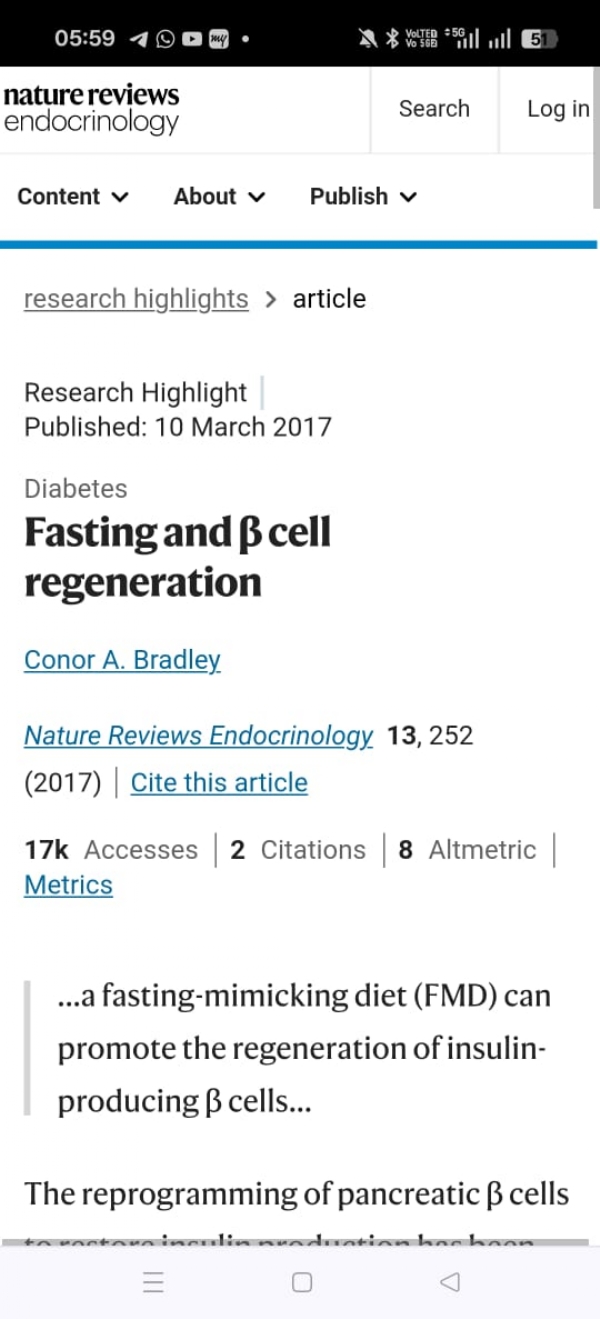
Experimental Evidence: Fasting and Ngn3 Activation. One of the most striking discoveries of the past decade has been the link between dietary interventions and pancreatic regeneration.
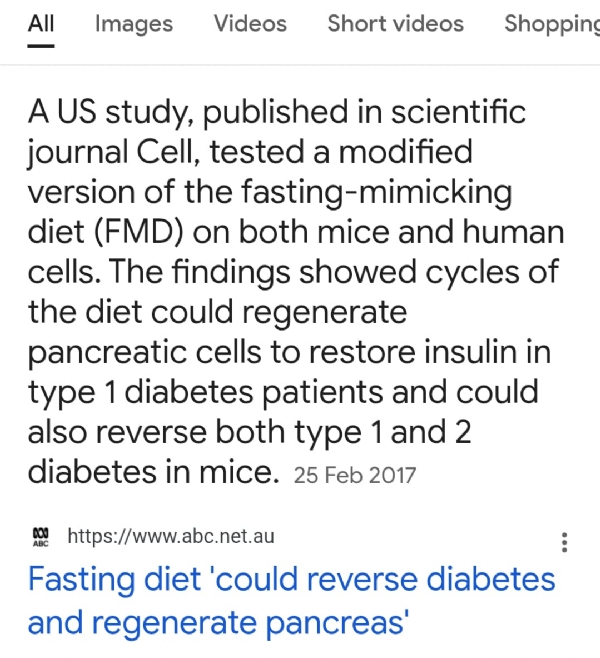
1. Fasting Mimicking Diet (FMD) Studies in Mice. In 2017, Cheng, Longo, and colleagues published a landmark paper in Cell. They demonstrated that cycles of a Fasting Mimicking Diet (FMD) in diabetic mice:
Suppressed growth signalling pathways (IGF-1, mTOR, PKA).
Induced a protective fasting state that promoted cellular reprogramming.
On refeeding, it triggered a surge in developmental genes, particularly Ngn3.
Led to the regeneration of insulin-secreting β-cells.
Restored normal glucose homeostasis even in mice with severe diabetes.
This was revolutionary: a simple nutritional intervention could switch on embryonic genetic programs in an adult organ.
2. Human Pilot Studies
Preliminary clinical trials of FMD in humans with prediabetes or type 2 diabetes revealed:
Reduction in fasting glucose and HbA1c.
Improved insulin sensitivity.
Increased C-peptide levels in some participants (suggesting improved endogenous insulin production).
Although direct evidence of Ngn3 activation in humans is limited, the metabolic improvements suggest similar pathways may be at play.
Molecular Pathways Linking Fasting to Ngn3. The mechanism by which FMD and fasting interventions activate Ngn3 can be broken down into phases:
1. Fasting Phase
Nutrient-sensing pathways suppressed: IGF-1, mTOR, and PKA are downregulated.
Cells shift into maintenance mode: reduced proliferation, increased autophagy, DNA repair, and stress resistance.
2. Refeeding Phase
When nutrients return, growth signals are reactivated.
This surge resembles embryonic conditions, providing the stimulus for dormant progenitor cells to express Ngn3.
3. Ngn3-Mediated Differentiation
Ngn3 re-expression drives progenitors to differentiate into endocrine cells.
These cells include insulin-producing β-cells that can repopulate islets and restore function.
Implications for Diabetes Treatment
1. Type 1 Diabetes
The autoimmune destruction of β-cells is the primary pathology.
If Ngn3-driven regeneration can replenish β-cells, there is potential for functional cure, especially if paired with immunomodulation to halt autoimmunity.
2. Type 2 Diabetes
In type 2 diabetes, insulin resistance is compounded by β-cell exhaustion.
Ngn3 reactivation may restore β-cell mass, improving insulin secretion capacity.
Combined with lifestyle interventions, this could lead to durable remission.
Challenges and Limitations
While the potential is extraordinary, several challenges remain:
Autoimmune Attack (Type 1 Diabetes)
Newly formed β-cells may be destroyed again unless the immune system is retrained.
Age-Dependent Plasticity
Younger organisms have more progenitor capacity; older adults may regenerate less effectively.
Safety Concerns: Overactivation of developmental pathways may risk uncontrolled proliferation or tumorigenesis.
Translation to Humans
Evidence in mice is strong; human evidence is preliminary and requires large, long-term trials.
Future Directions
Combination Therapies
Pairing Ngn3 activation strategies (via diet or pharmacology) with immune therapies for type 1 diabetes.
Small Molecules or Gene Editing
Developing drugs that mimic fasting signals and directly activate Ngn3 without dietary restriction.
Stem Cell Therapies: Engineering stem cells to overexpress Ngn3 for transplantation into diabetic patients.
Personalised Nutrition Programs
Tailoring FMD cycles based on genetic and metabolic profiles to optimize β-cell regeneration.
Conclusion: Neurogenin-3 stands as the master regulator of pancreatic endocrine differentiation, a gene that writes the blueprint for insulin-secreting islet cells. Once thought to be active only in the embryo, it is now recognised that Ngn3 can be reawakened in adulthood under specific stress and refeeding conditions, such as those induced by fasting-mimicking diets.
Animal studies have proven that Ngn3 reactivation can regenerate β-cells and reverse diabetes, while early human data show significant metabolic benefits, paving the way for future breakthroughs. Although challenges remain—autoimmunity, limited regenerative capacity in older adults, and translational gaps—the promise of Ngn3 as a therapeutic target is immense.
If harnessed effectively, Neurogenin-3 may allow humanity to move from managing diabetes to curing it, transforming millions of lives worldwide.
*Dr. Devan is a Manguluru-based ENT specialist and author.
Hindusthan Samachar / Manohar Yadavatti