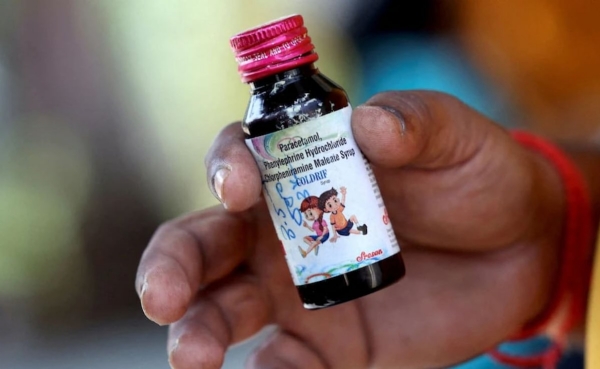
Washington/Delhi, October 11(HS): The US Food and Drug Administration (FDA) confirmed on Friday that toxic cough syrups linked to the tragic deaths of children in India have not been shipped to the United States, providing some relief amid ongoing concerns over contaminated medicines. The dangerously high levels of toxic diethylene glycol detected in locally sold cough medicines, including the banned brand Coldrif, have been blamed for the deaths of 17 children under the age of five in India over the past month.
The World Health Organization (WHO) highlighted a regulatory gap in India’s oversight of syrup medicines, prompting Indian authorities to issue warnings against two additional brands following these fatalities. The Central Drugs Standard Control Organization of India informed the US FDA that these contaminated products were not exported internationally, ensuring that US consumers remain unaffected.
The FDA said it is closely monitoring the situation and urged pharmaceutical manufacturers to guarantee the safety and high quality of drugs available in the US market. This development underscores the critical need for tighter drug regulations and international vigilance to prevent similar public health crises.
Hindusthan Samachar / Jun Sarkar